Biography
Biography: Alberto Abad
Abstract
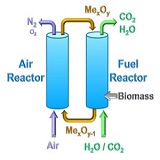
Bioenergy and Carbon Capture and Storage (BECCS), is an interesting option to remove CO2 from the atmosphere, thus mitigating the CO2 emissions from the use of non-renewable sources, i.e., fossil fuels. BECCS has been identified as a relevant measure toachieve the target enforced by the United Nations Framework Convention on Climate Change (UNFCCC) in the Paris Agreement:To limit the increase in the average world temperature to 2ºC above pre-industrial levels. However, implementing CO2 capturein bioenergy through common technologies has the drawback of high economic and energetic costs. In this sense, the Chemical Looping Combustion (CLC) technology allows inherent CO2 capture at low cost during combustion. The benefits of CLC are based on avoiding the costly separation steps required in commercial CO2 capture processes, e.g., CO2 separation in flue gases or O2 production for oxy-fuel combustion, by the use of an oxygen carrier. The purpose of the oxygen carrier, usually a particulate metal oxide, is to transfer oxygen from air to fuel in order to avoid the direct contact between them. Thus, the oxygen carrier provides the oxygen required for combustion in the so-called fuel reactor. The oxygen carrier is later regenerated by air in the air reactor. The most common design of a CLC unit includes two fluidized bed reactors, being those mentioned fuel and air reactors with the oxygen carrier continuously circulating among them. CLC has been widely investigated for the use of gaseous fuels and coal but the interest of using biomass has recently increased considering that negative CO2 emissions would be possible. The objective of this work is to contributeto the development of biomass combustion by CLC evaluating the use of new and highly reactive Mn-based materials as oxygen carriers. Experiments were performed in a continuous 500 Wth CLC unit at Instituto de Carboquimica (ICB-CSIC), consisting oftwo interconnected fluidized-bed reactors. After determination of both gas streams composition, the performance of the CLC process was assessed by calculating the CO2 capture rate and the combustion efficiency as a function of the operating conditions. During the experimental campaign, the temperature in fuel reactor and the circulation rate of the oxygen carrier were varied. In general, CO2 capture rates close to 100% were obtained, which increased with temperature. In addition, high values of combustion efficiency were obtained. When the combustion was incomplete, the major unburnt compounds from the fuel reactor were H2, CO and CH4. Likely, these unburnt gases could proceed from the volatile matter as a high conversion of char would be expected. Interestingly, the amount of tar detected was low and they do not contribute significantly to the combustion efficiency.